PerioDontaLetter

Histologic Evaluation of an Nd: YAG Laser- Assisted New Attachment Procedure in Humans
Raymond A. Yukna. DMD, MS*
Ronald L Carr, DDS** Gerald
H Evans. DDS”
This report presents histologic results in humans following a taser-assistec/new attachment procedure (LANAP®) for the treatment of periodontal pockets. Six pairs of single-rooted teeth with moderate to advanced chronic periodontitis associated w/th sub gingiva/ calculus deposits were treated. A bur notch was placed within the pocket at the clinically and radiographically measured apical extent of calculus. All teeth were scaled and root planed with ultrasonic and hand scalers. One of each pair of teeth received treatment of the inner pocket wall w/tha free-running pulsed neodymium:yttrium-aluminum-gamet (Nd:YAG) laser to remove the pocket epithelium, ano* the test pockets were laseda second time to seal the pocket. After 3 months, all treated teeth were removed en bloc for histologic processing. LANAP®-treated teeth exhibited greater probing depth reductions andclinical probing attachment level gains than the control teeth. All LANAP®-treated specimens showed new cementum and new connective tissue attachment in and occasionally coronal to the notch, whereas five of the six control teeth had a long junctional epithelium with no evidence of new attachment or regeneration. There was no evidence of any adverse histologic changes around the LANAP® specimens. These cases support the concept that LANAP® can be associated w/th cementum-mediated new connective tissue attachment and apparent periodontal regeneration of diseased root surf aces in humans. (Int J Periodontics Restorative Dent 2007;27:577-587.)
Regeneration of the supporting tissues of the teeth is a primary goal of periodontal therapy. Whereas clinical results and animal histology suggest that n ew connective ti ssue atta ch m ent (CTA) as well as regeneration of cementum (CEM), periodontal ligament (PDL), and alveolar bone (AB) can occur on human teeth affected by periodontitis as a result of several treatment approaches, histologic evidence in humans of successful cases and successful treatments is limited.1
The 1996 World Workshop in Periodontics established specific histologic c liter ia for proof of reg en erat ion. Experimental teeth must have loss of CTA and AB associated with periodontitis. In addition, subgingival and/or subcrestal calculus must be present at the time of surgery so that a notch ca n be ma de i nto th e root at th e apical extent of calculus. Proof of new attachment is demonstrated by new CEM and CTA, and regeneration is evidenced by the presence of new CEM, PDL, and AB coronal to the apical extent of the notch. Most treatments that show proof of new attachment and regeneration are associated with surgically implanted devices or materials.1’21
‘Professor, Department of Periodontics, Louisiana State University School of Dentistry, New Orleans, Louisiana.
“FVofessor, Department of Oral Pathology, Louisiana State University School of Dentistry, New Orleans, Louisiana.
Correspondence to: Dr Raymond A. Yukna, Advanced Periodontal Therapies, University of Colorado Dental School, 13065 East 17th Place, Room 111, P.O. Box 6508, MS F847, Aurora, CO 8004S; fax; 303-724-0162; e-mail: ray.yukna@uchsc.edu.
Sulcular/pocket epithelium removal has been the basis or foundation of subgingival curettage (CUR), the excisional new attachment procedure (ENAP), and the replaced flap/modified Widman fla p procedu re to set up an environment for new CTA.22-25 However, elimination of pocket epithelium by CUR, ENAP, or other internal- bevel incision designs appears nearly impossible.26
Procedures limited to treating the soft tissue wa II of periodonta I pockets such as CUR and ENAP would not be expected to influence new bone formation to a ny great degree but hope-fully would lead to healing with a CTA ratherthan a long junctional epithelium (UE). Almost all available human histologic evidence to date demonstrates healing by an UE with no or minimal CTA27
Interest in neodymiurrvyttrium:aluminum-garnet (Nd:YAG) laser use in periodontics is increasing. Several papers have suggested favorable results with its use in the treatment of periodontal pockets.28-30 A procedure called laser ENAP has been promoted in trade journals with examples of radiographic bone regeneration.31’32 Referred to as the laser-assisted new attachment procedure (LANAP®) inthis report, this technique of pocket therapy has recently been approved by the US Food and Drug Administration (FDA 51 Ok clearance K030290).
In clinical case reports LANAP® has demonstrated improved clinical measurements a nd some radiographic evidence of bone regeneration in the areas treated.33-37 However, it is not known what tissues constitute the new healed interface between the soft tissues and the tooth root. Also, there is some evidence that the use of lasers in periodontal pockets may damage root surfaces,38-10 adversely affect the adjacent alveolar bone,50,51 or cause undesirable pulpal changes.48,49 Clinical case reports have reported favorable results, but there is no human histologic proof of the nature of the healing following LANAR The purpose of this paper is to report histologic wound healing following use of LANAP® surgery for periodontal pockets.
Method and materials
Dental radiographs of patients assigned to the Postgraduate Periodontics Clinic, Louisiana State University (LSU), were screened for the presence of teeth that had isolated moderate to severe periodontal involvement (probing depths and clinical probing attachment loss of 5 to 9 mm with bleeding on probing and evident subgingival calculus). Teeth that had been treatment planned by dinicians in the Oral Diagnosis and/or Prosthodontics departments for extraction as part of the overall restorative treatment plan were induded in the study. Subjects had to provide two single rooted teeth with similar periodontal involvement forthe study and signed an LSU-approved consent form prior to beginning the study.
Preoperatively, the subjects received occlusal adjustment/odontoplasty to reduce ooolusal forces on the experimental teeth, and study teeth were splinted to neighboring teeth with an extracoronal bonded splint (Ribbond, Ribbond Inc). Scaling and root planing were performed on othe r teeth in the same segment (not the treatment teeth), and general supragingival prophylaxis was provided forthe rest of the mouth.
Documentation consisted of dinical photographs, radiographs with stent and grid (Fig 1), modified Gingival Index (mGI),52 Quigley-Hein Plaque Index (PI),53 and clinical mobility evaluation.54 Clinical measurements were made from the cementoenamel junction (CEJ) to the free gingival margin, from the CEJ to the base of the pocket, from the CEJ to the apical extent of clinically and radiographically evident calculus, and from the CEJ to the mucogingival junction. Bleeding on probing (BOP) was also assessed.
Appropriate laser safety precautions were used. Under regional local anesthesia, a quarter round bur notch was placed at the clinically and radiographically measured apical extent of calculus as carefully as possible. One of each pair of teeth randomly received Nd:YAG laser treatment (Periolase, Millennium Dental Technotogies) of the i nner pocket wa II to remove the crevicular epithelium around the necks of the study teeth, relax the gingival collar, and expose more of the contaminated root surface. The fibertipof the laser was directed parallel to the root surface and was moved laterally and apicalry along the pocket wall, eventually reaching dose to the base of the pocket. The laser settings for this first pass were 3 W, 150-us pulse duration, and 20 Hz. Once the epithelial lining was removed, root debridement was accomplished coronal to the area of the calculus reference notch with ultrasonic (EMS Piezon 4C0, EMS) and hand instrumentation.
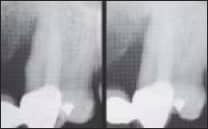 Fig 1 Radiographs of a 54-year-old man with deep infratony defeca on the mesial of torn maxillary canines.
Fig 1 Radiographs of a 54-year-old man with deep infratony defeca on the mesial of torn maxillary canines.
Fig 1a(left} Mesial defecton the right canine (left), treated iw’th LANAR demonstrates a radiographic increase in tone den¬sity and apparent fiil of defect at 3 months after treatment (right).
 Fig lb Mesialdefectonrhe left canine (left), treated iMth scaling and root planing without laser demonstrates little change in the tony defect contour or Done density after3 months.
Fig lb Mesialdefectonrhe left canine (left), treated iMth scaling and root planing without laser demonstrates little change in the tony defect contour or Done density after3 months.
No attempt was made to remove any soft/granulation tissue with the mechanical instrumentation. The pocket contents of the test teeth were lased again (4 W, 635-us pulse duration, and 20 Hz) to help achieve a solid fibrin clot and form a pocket seal. The control teeth received all of the aforementioned treatment except for the lasertherapy No sutures were used, and triple antibiotic ointment and a light-cured dressing (Barrica id, Dentsply/Caulk) were placed cnall teeth. Al I patients were provided with nonsteroidal anti-inflammatory medications, doxycycline (100 mg daily for 10 days), and 0.12% chlorhexidine rinses (to be used twice daily).55
After3 months, a second surgical procedure was performed to remove the experimental tooth roots en bloc according to methods described pre-viously4-9-56-57 For all teeth this was a single proximal area. The body of each tooth root was bisected longitudinally in a faciolingual plane, with the clinician attemptingto keep at least half ofthe root diameter attached to the area of interest. A small interproximal wedge oftissue and a section of root approximately 5 mm wide, 7 mm long, and 5 mm thick was removed.
Once the desired specimens were completely freed, they were gently and atraumatically removed, rinsed gently in sterile saline, and placed in 10% neutral buffered formalin. The residua I defects were reconstructed, and after an appropriate healing period, the patients were referred for prosthetic replacements.
Essed by the LSU School of Dentistry Research Histology Laboratory, where they were decaldfied, embedded in paraffin so as to obtain longitudinal mesiodistal serial step sections, serially sectioned at 7 urn in the area of the notch, and stained with hematoxylin and eosin. The three most central 200-um serial step secticns were blindly and randomly evaluated for the nature ofthe healed tissues—specifically the presence and length of new CEM, new CTA, new AB, and healed junctional epithelium relative to the apical extent of the calculus notch. Histomorpho-metric measurements were made by an oral pathologist (RLQ using an eyepiece grid on the microsoope. Root resorption, ankylosis, pulpal changes (where pul p tissue was visible), and the degree of inflammation were also evaluated. Mean values for the three sections of each tooth were used for linear measurements.
Results
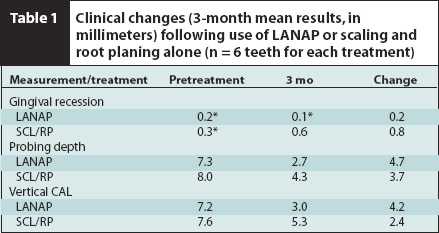
LANAP® = jlaser-assisted new attachment procedures; SCL/RP = scaling and root planing;
CAL = clinical attachment level.
*Coronal to cementoenamel junction.
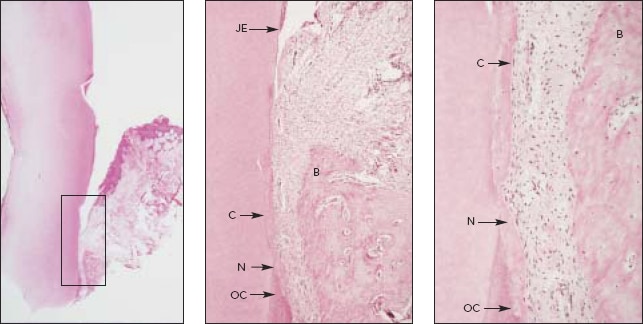
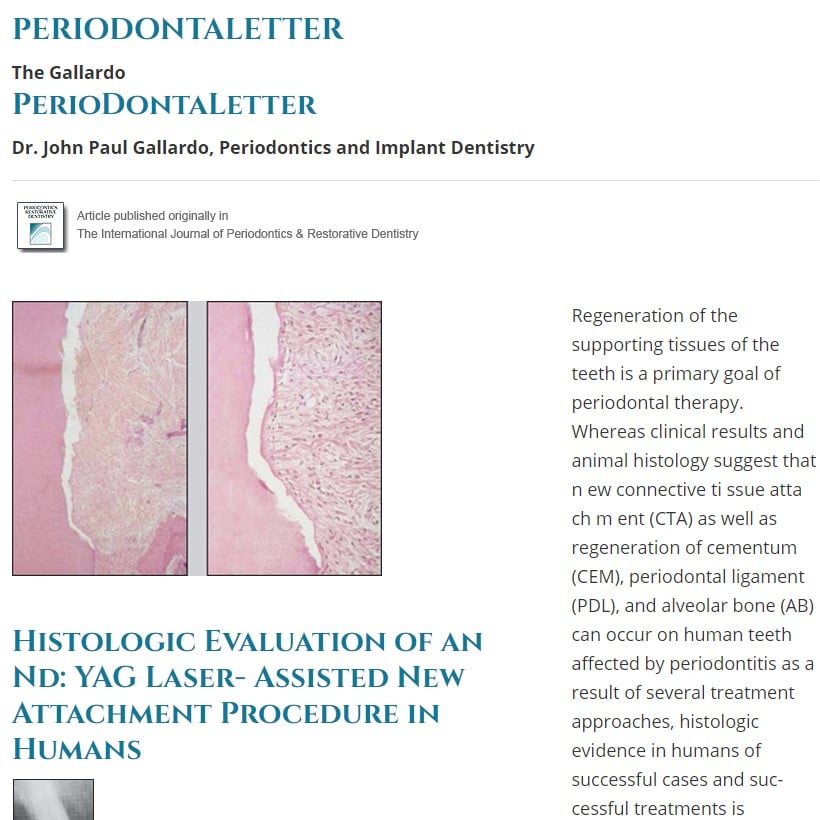
Fig 2 Histologic views of LANAP®-treated maxillary right canine from Fig 1a (hematoxylin & eosin). fleftJ Low-power view (Xl) with box around area of interest, (center,! Medium-power view (X 16) showing calculus notch (N) with new cementum (C) in and coronal to the notch and old cementum (OC) apical to trie notch, apical extent of junctional epithelium (JE), and newbone (B) adjacent to tfie notch, (right,) High-power view (X 40) of notch area demonstrating new cementum (C) filling tfie notch (N) and extending coronally, old cementum apical to the notch (OC) covered by new cementum, new alveolar bone (B), and new periodontal ligament and gingival fibers attached to the tooth.
Clinical results are presented in Table 1. mGI, PI, and BOP were improved on all testand control teeth. Total energy applied to the test pockets ranged from 14 to 25 J/mm of probing depth (mean 19 J/mm).
All six LANAP®-treated specimens showed new CEM and new CTA in and occasionally coronal to the notch (Figs 2 to 5). In two specimens, the notch was within the infrabony pocket (sub-crestal) and the new CEM and new
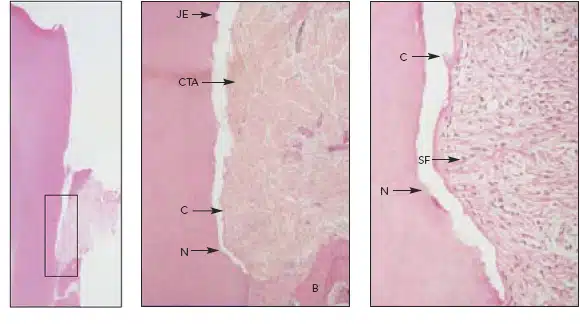
Fig 3 LANAP®-treated mandibular left second premolar of a 48-year-old man vwth an infrabonydefect (hematoxylin & eosin). fleftJ Low-power view (X1) outlining the area of inter¬est, (center and righlj Medium-power (X16) and high-power (X 63) views showing the calcu¬lus notch (N), th/’n layer of new cementum (C) in and coronal to the base ofthe notch, junc¬tional epithelium (JE) at the coronal level, newCTA with Sharpey fibers (SF), and newbone (B) adjacent to the notch. (Cementum is artificially separated from tooth.)
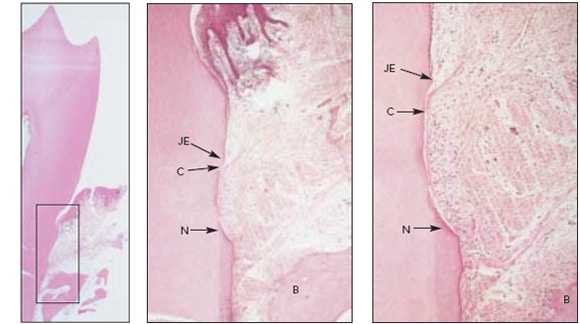
Fig 4 LANAP®-treated premolar w/th calculus notch coronal to bone crest (hematoxylin & eosin). (\eh) Low-power overview (X1) with box around area of interest, (center and right,) Medium-power (X 10) and high-power (X25) views with new cementum (C) in and coronal to the base of the calculus notch (N). The apical extent of the junctional ep/’the/jum (JE) stops near the coronal limitof new cementum. B = alveolar bone.
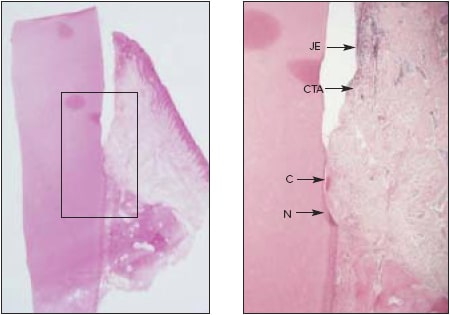
Fig 5 Canine tooth with calculus notch corona/ to bone crest treated with LANAP® (hematoxylin Steosin). (left) Low-power (X1 ) view with box around area of interest, (right) Medium-power fx 10) Mew showing new cementum (Q in and coronal to the calculus notch (N). New CTA is evident between new cementum and trie apical extent of the junctional epithelium (JE).
*Mean amount, in millimeters, from micrometer readings.
LANAP® = laser-assisted new attachment procedure, SCL/RP= scaling and root planing; CTA = connective tissue attachment.
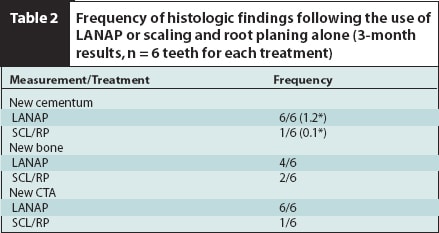
CTA were adjacent to new AB, technically showing periodontal regeneration. Rve of the six control teeth had an UE, with no evidence of new attachment or regeneration (Figs 6 and 7). One control specimen did show a small amount (0.1 mm) of new CEM and CTA. There was no evidence of any adverse histologic changes to the root surface or the pulp of any of the teeth. Histologic results are presented in Table 2.
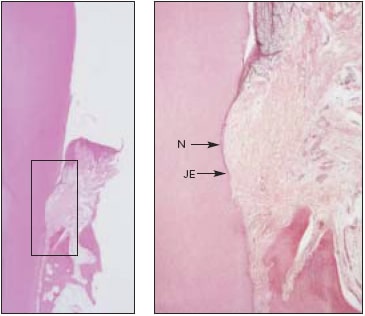
Fig 6 Control tooth treated with scaling and root planing without laser application (hematoxylin &eosin). (left) Low-power (x 1) view with box around area of interest, (right) Medium-power (X10) image showing calculus notch (N) with no evidence of new CEM, new AS, ornewCTA The junctional” epithe-lium (JE) extends to the apical extent of the notch.
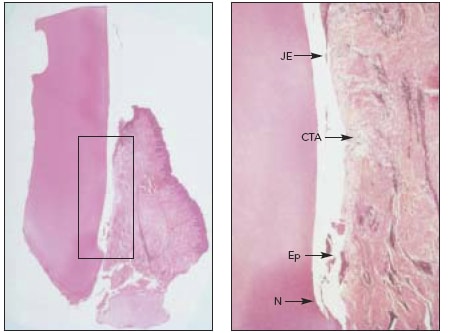
Fig 7 Manc/ibu/arpremo/ar that received control treatment (scaling and rootplaning alone) (hematoxylin & eosin). (left) Low-power fx 1) view with box around area of interest, (right) Medium-power fx 10) view demonstrating lack of good tissue contact with root even though CTA is present between the calculus notch (N) and junctional epithelium (JE). No new CEM is evident, and some epithelial islands ftp) are present at the depth of the pocket and as islands within the connective tissue.
Discussion
This human histologic report demonstrates favorable histologic healing with the useof the free-running pulsed Nd:YAG laser used in a specific patented technique of LANAP® Apparent periodontal regeneration (CEM, PDL, AB) on a calculus- and plaquecontaminated area of the root was seen on two of the test teeth, and CEM-mediated new attachment was evident on the other four laser-treated teeth. Similar periodontal healing in humans has been shown with other surgical techniques.2-21 , 58-60
The histologic assessment was based primarily cn preserxe/absence criteria but also induded linear measurements of new CEM length. As with the control teeth in thts report, the literature demonstrates consistent and almost un iversal hea ling by U E following scaling and root planing, gingival curettage, and open flap debridement procedures2,3,56-57,59-62 and variable histologic results with bone replacement graft materials and miscellaneous regenerative agents on contaminated rootsurfaces.3-21,59,60,61,63-68
Comparison of the results of this study with those from a study that used demineralized freeze-dried bone allografts2 suggests essentially equivalent histologic results using the LANAP® procedure. In this study new CEM was seen in 100% of the cases versus 77% of the cases in the Bowers et al 2 study; new CEM length was the same (1.2 mm) as in Bowers et al; and the frequency of new CTA was 100% versus 68% for Bowers et al. It should be noted that the number of specimens was larger in the study of Bo we rs et a I.
Treatment allocation could not be concealed from the therapist, as he had to use the laser on one tooth and not the other. Treatment (laser or no laser) was allocated according to a random code after all preliminary measurements and procedures, including placement of the calculus notch, had been completed.
Accurate placement and evaluation of the calculus notch presented severa I chal lenges Since no fla ps were reflected, direct visualization of thecalculus was not possible. Positioning of the notch was based on repeated measurements from the CEJ to the clinically detectable calculus and evaluation of calculus when it was evident on the radiographs. The appropriate “depth” was marked on the shank of the quarter round bur, and the notch was placed as carefully as possible. Again, because no flap was reflected, the depth of the notch into the root was confined to the lateral part of the bur head and was necessarily limited. It is true that there is no direct way to guarantee that the notch was actually placed in calculus, but this was the most tedious and difficult part of the entire procedure because the depth of the bur placement and therefore the depth of the notch was based on clinical and/or radiographic detection of calculus. Since clinical and radiog raphic detection of ca leu lus leads to many false-negative but nofalse-positive results, it is felt that this was as accurate as could be accomplished with the dosed procedure employed. In addition, if any error was made it was to plaoe the notch more coronally to be sure that it was in calculus and/or contaminated root surface. On the histologic slides, the position of the notch was verified by using the clinical measurements related to the CEJ or biopsy-related landmarks.
It should be emphasized that the LANAP® is a combined therapy using a patented protocol (US patent #5,642,997) that includes several aspects: occlusal adjustment, splinting when needed, systemic and topical antibiotics, laser use for surgical pocket epithelium removal, scaling and root debridement, and laser use for tissue stabilization (welding) against the tooth surface withafibrin clot. Use of the laser without attention to these other aspects may not yield the results reported here. It should be recognized that LANAP® is a single-treatment surgical procedure. Since tissue is surgically removed from the lining of the pocket with the laser (rather than with a seal pel) and occlusal adjustment is an integral part of the protocol, it would appear that only qualified clinicians can legally perform the treatments in most locales.
In condusicn, this study demonstrated consistently positive histologic responses in periodontal pockets in humans treated with the LANAPCEM mediated new attachment and occasionally apparent periodontal regeneration following a specific protocol with a free running pulsed Nd:YAG laserwere demonstrated.
Acknowledgments
This study was supported by Millennium Dental Technologies. which provided the laser, trainning and funding. The evaluations and conclusions made are solely those of the authors. The authors wish to acknowledge the histologic processing provided by Joanne Canale: the clinical assistance of Elizabeth Mayer. ROH. Stephanie Well. CDA. RDH. and Susan Bllllot. RDH; ard the efforts of Julie Behan. RHI A. and Aubrey quinn in preparing Ihis manuscript.
References
- Garrett S. Periodontal regeneration around natural teeth. Ann Perlodontol 1996:1: 621-666. 668.
- BowersGM.ChadroffB.CamevaleR.et3l. Histologic evaluation of a new attachment apparatus formation In humans. Part III. J. Perlodontol 1989;60:6B3-i93.
- Bowers G. Chadroff B. Camevale R. et al. Histologic evaluation of new human attachment apparatus In humans. Part I. J Perlodontol 1989;60:664-67J.
- YuknaRA. Mellonlg JT. Histologic evaluation of periodontal hea ling It humans following regenerate therapy with enamel math* derivative. A 10-case series. J Perlodontol 2000;71:752-759.
- Mellonlg JT. Enamel matrix derivative for periodontal reconstructive surgery: Technnical and clinical and histologic case report. Int J Periodontics Restorative Dent 1999;19:9-19.
- Mellonlg JT. Human histologic evaluation of a bovine-derrived bone xenograft In the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent 2000;20:19-29.
- CameloM. Nevlns ML. Schenk RK. Slmlon M. Rasperlnl G. Lynch SE. Nevlns M. Clinical, radiographic, and histologic evaluation of Km an periodontal defects treated with Blo-Oss and BioGide. Int J Pertodontlcs Restorative Dent 1998:18: 321-331.
- Sculean A. Chlantella GC. Windisch R Donod N. Clinical and histclogic evaluation of human Intrabony defects treated with and enamel matrix protein derivative (Emdogain). Int J Periodontics Restorative Dent 2000:20:375-381.
- YuknaRA.SalinasTJ.CarrRF.Perlodontal regeneration following use of ABM/P-15. A case report. Int J Periodontics Restor Dent 2002:22:146-155.
- Camels M. Nevlns M. Lynch S. Schenk R. Slmlon M. Nevlns M. Periodontal regeneration with an autogenous bone-Blo-Oss composite graft and a Blo-Glde membrane. Int J Periodontics Restorative Dent 2001;21:109-119.
- Winisch P. Sculean A. Klein F. et al. Comparison of cilniral. radiographic, histometric measurements following treatment with guided tissue regeneration or enamel matrix proteins In human periodontal defects. J Pertodontol 2002:73: 409-417.
- Nevins ML. Camelo M. Lynch SE. Schenk RK. Nevins M. Evaluation of periodontal regeneration following grafting Intra-bony defects with Blo-Oss Collagen: A human histologic report. Int J Periodontics Restorative Dent 2003;23:9-17.
- Sculean A. Windisch P. Keglevich T. Chlantella G. Gera I. DonosN.Clinical and histologic evaluation of human intrabony defects treated with an enamel matrix protein derivative combined with a bovine derived xenograft. Int J Periodontics Restorative Dent 2003:23:47-55.
- Camelo M. Nevins M. Schede R. Lynch S. Nevlns M. Periodontal regeneration in human Class II furcations using purified recombinant human platelet-derived growth factor-BB (rhFOGF-BB) with bene allograft. Int J Periodontics Restorative Dent 2003:23 231-225.
- Cochran DL. Jones A. Mellonlg JT. Sdioorfleld J. Khg G. Periodontal regen-eratkn with a combhatlon of enamel matrix proteins and autogenous tone grafting. J Perlodontol 2003:74:1269-1281.
- NevinsM. Camelo M. Nevtis ML Schenk R. Lynch S. Periodontal regeneration In humans using recombinant human platelet-derived growth factor-BB (rhPDGF-BB) and allogenic bone. J Perlodontol 2003;74:1282-1292.
- Hartman G. ArnoH R. M Ills M. Cochran D. Mellonlg JT. Clinical and histologic evaluation of anorganic bovine bone ccllagen with or without a collagen barrier. Int J Periodontics Restorative Dent 2004:24: 127-135.
- Sculean A. Wilndisch P. Chlantella G. Human histobgt: evaluation of an Intrabony defect treated with enamel matrix derivative, xenograft, and GTR. Int J Periodontics Restorative Dent 2004:24: 326-333.
- Sculean A. Windisch P. Keglevich T. Gera I. Clinical and histologic evaluation of human Inflatory defects treated with an enamel matrix protein derivative combined with a bloactlve glass fee the treatment of ntratony periodontal defects n humans. Int J Periodontics Restorative Dent 2005:25:139-147.
- Ma(ioubZ.BobboM. ADyehF.Cardiologi G.. Two patterns of histologic healing In intrabony defect following treatment with enamel matrix derivative: A human case report. Int J Periodontics Restorative Dent 2005:25:283-294.
- Mellonig JT Hlstobgi: and clinical evaluation of an allogenic bone matrix for the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent 2006:26:561-569.
- Yukna RA, Bowers GM. Lawrence JJ. Fedl PF Jr. Aclinical study of healing in humans following the treatment new attachment procedure. J Perlodontol 197647:696-700.
- YuknaRA. Aclinical and histologic study of healing following the excisional new attachment procedure in rhesus monkeys. J Pertcdontd 1976:47:701-709.
- Echeverrta JJ.CaffesseRG. Effects of gingival curettage when performed 1 month after root Instrumentation. A blometnc evaluation. J On Perlodontol 1983:10: 277-286.
- Ramflord SP. CaffesseRG. Morrison EC. et al. Four modalities of periodontal treatment compared over 5 years. J Clin Perlodontol 1987;14:445-452.
- Liltch JM. OlearyTJ. Kafrawy AH. Pocket epithelium removal via crestal and subcrestal scalloped interna I bevel Incisions. J Perlodontol 1984;55:142-148.
- Cobb CM. Non-surgical pocket therapy: Mechanical. Ann Periodontal 1996:1: .143-490.
- MyersTD. Lasers In dentistry: Their application In clinical practice. J Am Dent Assoc 1991:122:46-.
- Gokl SI. Vtlardl MA. Pulsed laser beam effects on gingiva. J Clin Perlodontol 1994:21:391-396.
- BenHatltY.BIumR.SevennC.MaquinM. Jabro MH.Tre effects of a pulsed ND:YAG laseron subgingival bacteria I flora and on cementum: An In vi.o study. J Clin Laser Med Surg 1996:14:137-143.
- Gregg RH. McCarthy DK. Laser ENAP for periodontal bone regeneration. Dent Today 1998:17:88-91.
- Gregg RH. McCarthy DK. Laser ENAP for peridental ligament (PDL)regeneratlon. Dent Today 1998:17:86-89.
- Nelll NM. Mellenlg JT Clinical efficacy of the ND:YAG laser for combination periodontitis therapy. Pract Periodontics Aesthet Dent 1997S(suppD:1-5.
- Merer A. Schoop U. Goharkhay K. et al. Treatment of periodontal pockets with diode laser. Lasers Surg Med 1998:22: 302-311.
- Radvar M. MacFarlane TN. MacKenzle D. Whltters CJ. Payne AP. Klnane DF. An evaluation of the Nd:YAG laser In periodontal pocket therapy. Br Dent J 1996:180:57-62.
- Liu CM. Hou LT. Wong MY. Lan WH. Comparison of Nd:YAG laser versus scaling and root planing n periodontal therapy. J Perlodontol 1990;70:1276-1282.
- WhteJM.GoodlsHE.RoseCL.Useoftte pulsed Nd:YAG laser for Intraoral soft tissue surgery. Lasers Surg Med 1991:11: 455-461.
- Coob CM.McCawley TK.Killow WJ.Aprellminary study on the effects of the ND:YAG laser on root surfaces and subgingival microflora h vivo. J Perlodontol 1992:63:701-707.
- Tewfik HM. Garnlck JJ. Schuster GS. Sharawy MM. Structural and functional changes of cementum surface following exposure to a modified Nd:YAG laser. J Perlodontol 1994:65:297-300.
- Morlook BJ. Pippin DJ. Cobb CM. Killoy W J. Rapley JW. The effect of Nd:YAG laser exposure on root surfaces when used as an adjunct to root planing. J Perlodontol 1992;63:637-641.
- Spencer P. Trylovich DJ. Cobb CM. Fhotoacoustc FTIR spectroscopy of lased cementum surfaces. J Perlodontol 1992; 63:633-636.
- Spencer R Cobb CM. McCollum NM. Wieliaka DM. The effects of CO; laser and Nd:YAG with and without water/air surface cooling on tooth root structure: Correlaticn between FTIR spectroscopy and histology. J Periodontal Res 1996:31: 453-462.
- Thomas D, Rapley JW, Cobb CM, Spencer P, Killoy WJ. Effects of the Nd:Yag laser and combined treatment on in vitro fibroblast attchment to root surfaces. J Clin Periodiodontal 1994;21:38-44
- Gopln BW. Cobb CM. Rapley JW. Killoy WJ. Histologic evaluation of soft tissue attachment to CO; laser treated root surfaces: An in vivo study. Int J Periodontics Restorative Dent 1997; 17:317-32 5.
- Trylovich DJ. Cobb CM. Pippin DJ. Spencer P. Kllky WJ. The effects of the Nd:YAG laseron in fibroblast ttach-ment to erdotoxtv treated root surfaces. J Perlodontol 1992:63:626-632.
- Radvar M.CreanorSLGllmourVTH.etal. An eva kia Don of the effects of an Nd:YAG laser on subgingival calculus, dentine and cementum. An In vitro study. J Clin Perfcdontol 1995;22:71-77.
- Malllet WA. Tomeck CD. Friedman S. Connective tissue response to root surfaces resected v/lth Nd:YAG laser or burs. Oral Surg Oral Med Oral Pathol 1996:82: 681-490.
- Tokita Y. Sunakawa M. Suda H. Pulsed Nd:YAG laser irradiation of the tooth pulp in the cat: I. Effect of spot laslng. Lasers Surg Med 2000;26:398-404.
- Sunakawa M. Toklta Y. Suda H. Pulsed Nd:YAG laser Irradiation of the tooth pulp in the cat: II. Effect of scanning laslng. Lasers Surg Med 2000;26:477-484.
- Krause LS, Cobb CM, Rapley JW, Killoy WJ, Spencer P Law Irradiation of bone. I. Art in vitro itudy concerning the effects of the CO; laser on oral mucosa and subjacent bone. J Periodontal 1997;68: 872-880.
- Fnesen LR. Cobb CM. Rapley JW. Forgas-ßrockman L SpencerP. Later vraduton of bone. IL Htafcng response foeowngreat-mervi by CO, and Nd:YAG lasers. J Pwwdoraol 1999:70:75-83
- Lobene RR. Weafcerford T, Ross NM. Lamm RA. MenakerL. Amodffied gingival index for use in clinical trials. Clin Prev Dent1986;8; 3-6.
- Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the diloromtthyl analogue of Victamine C. J Periodontol 1970:4141-43.
- Miller SC. Textbook of Periodontia, ed 3. Ptiiladefcnia: Blakitton. 1950:125.
- Sani M. Newman UG. Anderson L. Matoska W. Otom&Corgel J. Safari C. Canica I enhancement of post-periodntal surgical therapy by exkiene gluconate mouthrirne. J Periodontol 1989:60:570-576.
- Dragoo MR. Regeneration of the Periodontal Attachment in Humans. Philadelphia; Lea & Febiger. 1981.
- Bowers G, Granet M, Stevens M. et al. Histologic evaluation of new attachment in humans. A preliminary report. J Peri-odontol 1985:56:361-396.
- Cole RT, Crigger M, Böiger G. Egelberg J, Selvig KA. Connective tissue regeneration to pencdontally diseased teeth. A histological study J Periodontal Res 19); 15: 1-9.
- Bowers G, Chadrofl B, Camevale R. et al histologic evaluation of new human attachment apparatus in humans. Part IL J Penodontol 1989.61 675-682
- Stahl SS. Froum S Human intrabony lesion responses to debridement, porous hydroxyapatite Implants and Teflon barrier membranes. 7 histologic case reports. J Clin Perlodontol 1991;18:605-610.
- Stahl SS, Froum SJ. Healing of human suprabony lesion treated with gukied tissue regeneration and coronally anchored flaps. Case reports. J Clin Periodontal 1991;18:69-74.
- Stelner SS. Crigger M. Egelberg J. Owneetrve tissue regeneration to periodontaty diseased teeth II. Hrstoiogk cbservabons of cases lolowmg replaced flap surgery. J. Peridental Res 198l;16: 109-116.
- Stahl SS, Froum S. Histologic healing responses in human vertical lesions following the use of osseous allografts and barrier membranes. J Clin Periodontal 1991;18:149-152.
- Shepard WK, Bahat O. Joseph CE. LoPicccJo P, Bemlck S. Human clinical and histological respomes to a Calcitite implant in intraosseous lesions. Int J Periodontics Restorative Dent 1986:6: 46-63.
- Stahl SS. Froum SJ. MstotegK and cfcnkal responses to porous hydroxylapatite Implants in human periodontal defects: Three to twelve monnths postinplantation. J Fnodontd 1987;S*:689-69S
- Nevins Mi, Camelo M. Nevins M. et al. Human histologic evaluation of bioactive ceramic in the treatment of periodontal osseous defects. Int J Periodontics Restorative Dent 2000;20:459-467.
- Harris RJ. Human histologic evaluation of a bone graft combined ith GTR in the treatment of osseous dehiscence defects: A case report. Int J Periodontics Restorative Dent 2000:20:511-519.
- Parodi R. LwrroG. Patnxco P, et al. Use of Emdogam m the treament of deep intrabony defects: 12-momh caracal results. Histologic and radigrafic evaluation on Int J Periodontics Restorative Dent 200020: S8S-595.